Clinical, precise, reliable
In vivo efficacy tests represent the moment when a product truly proves what it claims.
Bio Basic conducts advanced clinical studies on selected volunteers to evaluate how the product performs under real-use conditions, closely observing the skin’s functional and sensory responses.
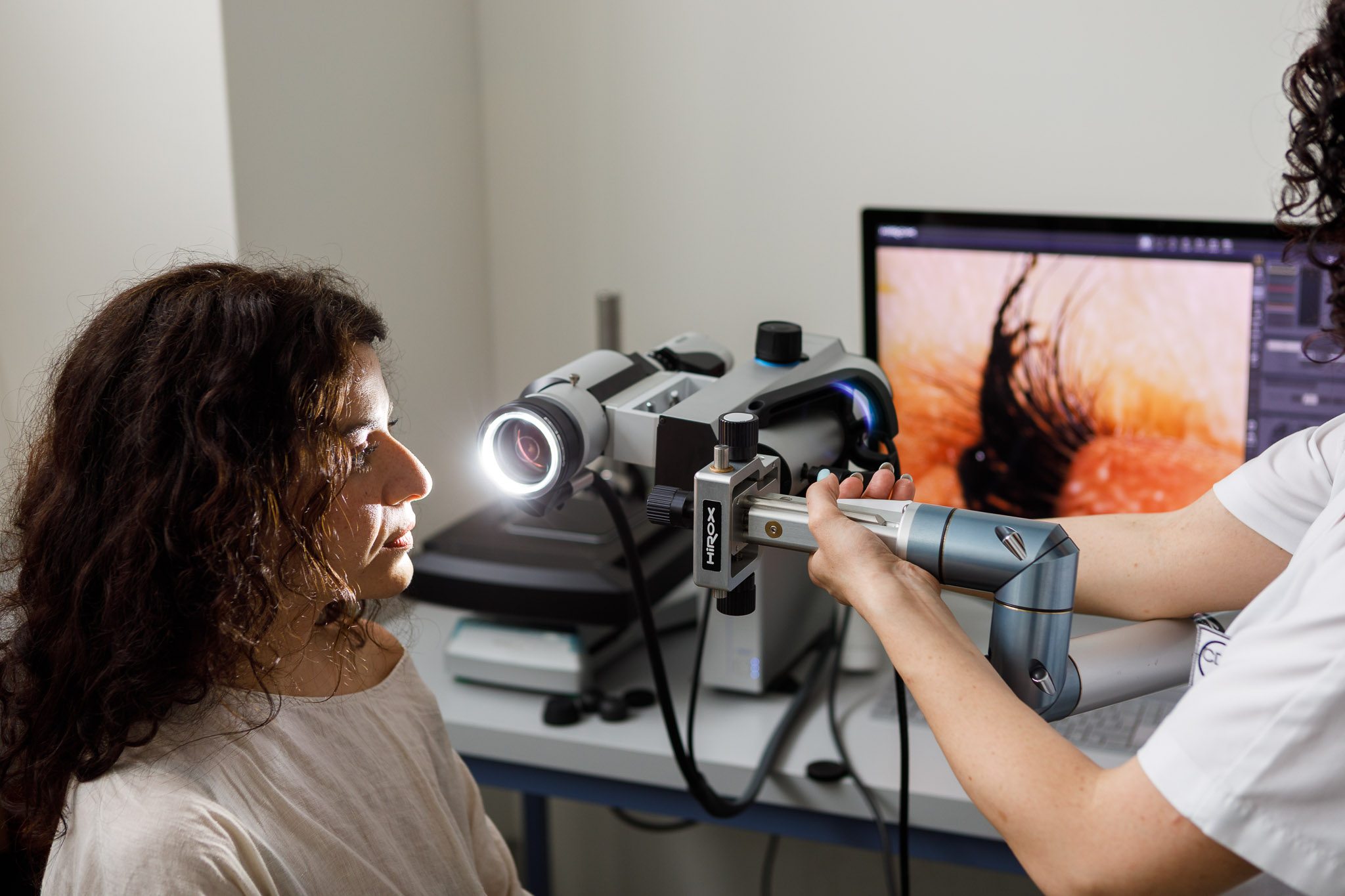
Through our specialist center CDC “Dermo-Clinical Research Center” and in collaboration with specialized medical facilities, our team objectively verifies claims such as hydration, soothing effect, anti-age action, sebum regulation, and many others.
The use of non-invasive CE-marked biomedical devices allows us to obtain precise, reproducible, and internationally recognized measurements.
Each study protocol is custom-designed in full compliance with EU regulations and the most recognised scientific guidelines. Our goal is to generate reliable clinical evidence capable of supporting product claims and enhancing its credibility on the market.


